Generation of ‘best-in-class’ MSCs for regenerative medicine
Our research platform focuses on obtaining clinically relevant numbers of MSCs obtained from bone marrow and adipose tissue by optimizing methods that improve survival post-isolation whilst also maintaining a high percentage of potent cells that are able to be used clinically. A multi-faceted approach is adopted to select therapeutically viable cells that can help predict favorable outcomes without any compromise to their differentiation or stemness properties. Our efforts in this research space will develop and support initiatives to advance regenerative medicine therapeutics with the goal to establish at the University of Arkansas a bioengineering center of excellence for MSC processing, manufacturing, and product development for several regenerative medicine clinical trials and applications.
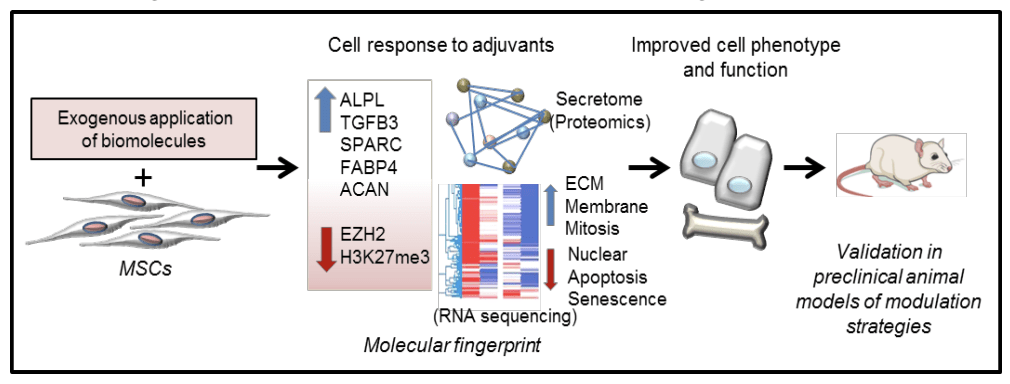
Epigenetic therapies for regenerative medicine
Given that chromatin states in stem cells are significantly different during undifferentiated (euchromatin) and differentiated (heterochromatin) states, our research focusses on epigenetic modulation of MSC chromatin with small molecules that can trigger specific transcriptional responses towards achieving desired stem cell phenotypes that facilitate regeneration upon transplantation. We are developing novel cell modulation epigenetic strategies as a viable method for enhancing and maintaining the osteoregenerative potential of mesenchymal stem cells intended for therapy.
Biomanufacturing of MSC-derived cellular products as therapeutics
Extracellular vesicles (EVs) derived from mesenchymal stem cells (MSCs) are promising therapeutic agents for a range of clinical disorders owing to their immunomodulatory and pro-regenerative functions. The beneficial effects of MSCs are largely mediated by EVs, and the direct application of EVs in clinical therapy has specific advantages over MSCs in that it circumvents the necessity of delivering live cells or maintenance of viability post-implantation. A major bottleneck in EV biomanufacturing is their scalability during development of product to achieve clinically relevant doses. Current practices in EV manufacturing rely on maximizing culture surface area, using feeder cells, conditioned media and substrates to improve EV secretion. Samsonraj Lab applies novel minimally manipulative methods that have the potential to be effective over otherwise harmful genetic alterations or immortalization methods in cell manufacturing.
Developing pre-clinical models for cellular therapy interventions
Animal models are vital tools for the preclinical development and testing of therapies aimed at providing solutions for several clinical disorders. Realizing the need for smaller and surgical models to study bone repair, the Samsonraj lab is directing efforts focused at devising versatile protocols in small animal models and three-dimensional cellular microsystems. The emphasis is to develop and advocate the utility of functional orthotopic bone formation models for the testing and validation of therapeutic interventions (such as growth factors, cells and biomaterials) intended for successful musculoskeletal regenerative therapies.
